Key Takeaways
- Retatrutide is a novel triple hormone receptor agonist targeting GLP-1, GIP, and glucagon receptors to effectively regulate hunger and energy expenditure, marking a significant advancement in obesity treatment.
- Clinical trials, including Phase 1 and Phase 2, have demonstrated Retatrutide’s substantial efficacy in weight reduction, with participants losing up to 24.2% of their starting body weight over 48 weeks, alongside improvements in glycemic control and lipid profiles.
- While gastrointestinal side effects were the most common, Retatrutide’s overall safety and tolerability profile align with other GLP-1 receptor agonists, and ongoing research aims to confirm its long-term safety and efficacy in chronic weight management and other health conditions such as NAFLD and obstructive sleep apnea.
Retatrutide for obesity is showing great promise in treating this condition, with clinical trials indicating significant weight loss. This article covers how Retatrutide works, its trial results, and its potential for obesity management.
Understanding Retatrutide
Retatrutide is a cutting-edge receptor agonist retatrutide specifically designed to combat obesity. Unlike traditional treatments, it uniquely targets the glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon receptors to regulate hunger and energy expenditure. This multi-receptor approach allows Retatrutide to effectively address the complexities of obesity, making it a powerful tool in weight management.
The advent of hormone receptor agonist retatrutide marks a significant advancement in the efforts to treat obesity. This drug influences multiple metabolic pathways, aiming to provide an all-inclusive solution for weight loss and energy regulation. This innovative approach has been the focus of extensive research, as scientists seek to understand and harness the full potential of this triple hormone receptor agonist.Mechanism of Action
Mechanism of Action
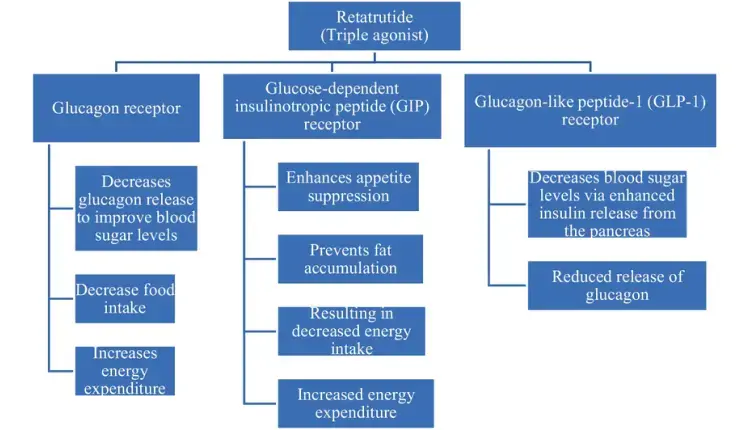
Retatrutide operates as a triple hormone receptor agonist, targeting the glucagon receptors, GIP, and GLP-1 receptors. These receptors play critical roles in regulating appetite and energy expenditure, making them prime targets for a comprehensive obesity treatment. Through its interaction with these pathways, Retatrutide effectively curbs appetite and fosters a balanced energy state, which is vital for sustainable weight loss.
This multi-targeted approach not only enhances the drug’s efficacy but also provides clinical proof of its potential benefits. For example, Retatrutide influences GLP-1 and GIP receptors, aiding in glucose regulation and insulin secretion, which further bolsters its role in metabolic health. Such detailed understanding of its mechanism underscores the importance of Retatrutide in addressing obesity from multiple fronts.
Development and Research
The journey of Retatrutide from concept to clinical application has been marked by rigorous research and collaboration among leading institutions. Key players like Yale Obesity Research Center and Velocity Clinical Research have been instrumental in studying the impacts of Retatrutide on weight loss and metabolic health. Their advanced research methodologies have paved the way for groundbreaking discoveries in obesity treatment.
Notably, the Yale Obesity Research Center has focused on understanding how Retatrutide influences obesity-related pathways, while Velocity Clinical Research has conducted significant trials to evaluate its efficacy. These efforts have not only provided valuable insights but also positioned Retatrutide as a promising candidate in the fight against obesity.
Clinical Trials Overview
Retatrutide has undergone extensive clinical trials to validate its effectiveness and safety as a treatment for obesity. These trials have been carefully structured to evaluate various aspects of its performance, ranging from initial safety reviews to its effects on weight loss and metabolic health. The multi-phase approach ensures a comprehensive understanding of Retatrutide’s potential benefits and risks.
Clinical trials for Retatrutide encompass multiple phases, each aimed at addressing specific research questions. From early-stage safety assessments to large-scale efficacy trials, these studies provide robust clinical proof of Retatrutide’s capabilities. This section examines the specifics of these trials, underscoring the major findings and their implications for obesity treatment.
Phase 1 Trials
Phase 1 trials for Retatrutide primarily focused on:
- Establishing the safety and optimal dosage range of the drug
- Involving small groups of healthy volunteers who received varying doses of Retatrutide to identify the most effective and safe dosage levels
- Monitoring for any adverse effects, which were generally mild and manageable
This initial phase was pivotal in laying the groundwork for subsequent efficacy trials, including a clinical trial.
Phase 2 Trials
Building on the safety data from Phase 1, Phase 2 trials aimed to evaluate Retatrutide’s efficacy in reducing body weight and improving metabolic health in obese patients. Sponsored by Eli Lilly, these trials involved larger cohorts and multiple dosages to comprehensively assess the drug’s performance. The results were encouraging, with notable weight loss noted across diverse dose groups.
One of the standout findings from Phase 2 was the substantial weight reduction achieved by participants. At the highest dose, participants lost more than 24% of their starting body weight within 48 weeks. Additionally, Retatrutide demonstrated significant reductions in HbA1c levels, indicating improved glycemic control. These outcomes underline the drug’s potential for not only weight loss but also for boosting metabolic health.
The Phase 2 trials also shed light on the safety profile of Retatrutide, with gastrointestinal issues being the most common adverse events. Despite these side effects, the overall benefits in terms of weight reduction and metabolic improvements underscore the drug’s potential as a transformative treatment for obesity.
Efficacy in Weight Reduction
Retatrutide has demonstrated remarkable efficacy in weight reduction, making it a promising candidate for obesity treatment. The drug’s ability to induce significant weight loss has been consistently observed in clinical trials, supporting its potential for long-term weight management. This section investigates the comprehensive outcomes of these trials, centering on the degree of weight loss and alterations in body composition.
The phase 2 trial results have been particularly impressive, with participants achieving substantial weight reduction. At the highest dose, an average weight loss of 24.2% over 48 weeks was recorded, highlighting the drug’s potent efficacy. Such results underscore Retatrutide’s promise as a comprehensive solution for obesity and rIllustration of metabolic health improvementelated metabolic disorders.
Mean Weight Reduction
The clinical trials for Retatrutide have consistently shown significant mean weight reduction among participants. Depending on the dosage, weight loss ranged from 7.2% to 24.2% after 48 weeks. This wide array of results suggests that Retatrutide can be customized to meet individual needs, positioning it as a flexible option for treating obesity.
In one study, more than a quarter of participants in the 12-mg dose group lost 30% or more of their baseline weight, showcasing the drug’s potential for dramatic weight reduction. These findings suggest that Retatrutide could be highly effective in long-term weight management, offering hope to those struggling with obesity.
Body Composition Changes
Beyond weight reduction, Retatrutide has also shown significant improvements in body composition. Clinical trials revealed reductions in both visceral and subcutaneous adipose tissue, contributing to overall weight loss and better health outcomes.
Additionally, participants experienced improvements in lean body mass ratios and body mass index, further enhancing the drug’s benefits for metabolic health.
Impact on Metabolic Health
Retatrutide’s impact extends beyond weight loss to include significant improvements in metabolic health. Clinical trials have demonstrated that Retatrutide treatment can lead to reductions in systolic and diastolic blood pressure, fasting glucose, and insulin levels. These improvements are essential for managing comorbidities related to obesity and enhancing overall health.
By addressing multiple metabolic pathways, Retatrutide offers a comprehensive solution for improving cardiometabolic measures. This section investigates the particular benefits noted in clinical trials, emphasizing glycemic control and enhancements in lipid profiles.
Glycemic Control
One of the most significant benefits of Retatrutide treatment is its impact on glycemic control. Clinical trials have shown clinically meaningful reductions in HbA1c levels across various dose groups, with some participants achieving reductions of up to 2.2%. This improvement in glycemic control is critical for patients with type 2 diabetes and those at risk of developing the condition.
Retatrutide has several benefits, including:
- Enhancing insulin sensitivity, as evidenced by significant reductions in fasting insulin levels
- Improving markers of pancreatic beta-cell function
- Contributing to better management of blood sugar levels and overall metabolic health.
Lipid Profile
Retatrutide has shown promising results in improving lipid profiles, including reductions in fasting triglycerides and improvements in cholesterol levels. Higher doses of Retatrutide led to a significant reduction in triglycerides by over 40% after 48 weeks. Additionally, improvements in blood pressure and low density lipoprotein cholesterol levels were observed, further supporting its role in enhancing cardiometabolic health.
Safety and Tolerability

The safety and tolerability of Retatrutide are essential considerations in its development as an obesity treatment. Retatrutide’s safety profile aligns with other GLP-1 receptor agonists, with gastrointestinal side effects being the most commonly reported adverse events. This section offers a summary of the safety data from clinical trials, spotlighting the types and severity of adverse events reported.
Despite the occurrence of side effects, Retatrutide’s overall safety profile is comparable to other incretin-based therapies. The advantages of substantial weight reduction and enhanced metabolic health overshadow these manageable side effects, positioning Retatrutide as a feasible option for obesity treatment.
Adverse Events
Gastrointestinal side effects were the most commonly reported adverse events in clinical trials of Retatrutide. These side effects were generally mild-to-moderate in severity and occurred primarily during the dose escalation period. Common symptoms included nausea, diarrhea, and vomiting, which subsided as the body adjusted to the treatment.
The incidence of adverse events underscores the importance of careful dose management and patient monitoring during treatment. Despite these side effects, the overall safety profile of Retatrutide remains favorable, with no reports of severe liver-related harm or other serious complications.
Long-term Safety
The long-term safety of Retatrutide will be further evaluated in longer-duration phase 3 trials. These trials will provide a more comprehensive assessment of the drug’s long-term efficacy and tolerability, particularly its potential to reduce long-term cardiac and liver-related harm from obesity.
Current research endeavors to confirm that Retatrutide remains a safe and effective option for long-term weight management.
Future Directions and Potential Applications
The future of Retatrutide is bright, with ongoing research exploring its potential role in long-term obesity treatment plans and its benefits in managing other health conditions. Combining glucagon receptor activation with GLP-1 or GIP-GLP-1 agonism could enhance its effects on energy intake and expenditure, offering new avenues for treatment. This section discusses potential paths for future research and potential applications of Retatrutide.
Retatrutide is also being investigated for its impact on cardiovascular risk factors and other metabolic health markers, including its potential role in the prevention or management of cardiovascular disease. Potential new applications include its use in combination therapies to enhance weight loss and metabolic outcomes, making it a versatile tool in the fight against obesity and related conditions.
Chronic Weight Management
Research is underway on Retatrutide’s potential role in long-term weight management. The drug’s ability to induce significant weight reduction and improve metabolic health makes it a promising candidate for long-term obesity treatment plans. Current studies aim to confirm its effectiveness in maintaining weight loss over prolonged periods, providing hope for sustainable weight management solutions.
Other Health Conditions
Retatrutide’s potential benefits extend beyond obesity treatment, showing promise in managing nonalcoholic fatty liver disease (NAFLD), obstructive sleep apnea, and even as a supportive measure for those who have undergone a liver transplant. In clinical trials, Retatrutide treatment normalized liver fat in 90% of patients with NAFLD over 48 weeks, highlighting its significant impact on liver health. This effect is crucial for patients with fatty liver disease, providing a therapeutic option that addresses a critical comorbidity of obesity.
Additionally, Retatrutide is being explored for its potential to improve outcomes for patients with obstructive sleep apnea. By reducing body weight and improving metabolic health, Retatrutide may alleviate symptoms of sleep apnea and enhance overall quality of life. The drug is currently in Phase III clinical trials for this condition, and if successful, it could offer a multi-faceted approach to treating obesity and its related health issues.
Summary
Retatrutide has emerged as a promising treatment for obesity, demonstrating significant weight reduction and improvements in metabolic health in clinical trials. Its unique mechanism of action, targeting GLP-1, GIP, and glucagon receptors, allows it to effectively regulate appetite and energy expenditure, leading to substantial weight loss. The clinical trials conducted so far have provided robust evidence of its efficacy and safety, with manageable adverse events.
As research continues, Retatrutide’s potential applications may expand to include long-term weight management and the treatment of other health conditions like NAFLD and obstructive sleep apnea. The ongoing studies aim to solidify its role in comprehensive obesity treatment plans, offering hope for a healthier future. Retatrutide’s journey from development to clinical success highlights the importance of innovative approaches in addressing complex health challenges.
Frequently Asked Questions
Meet the Author

Bradley Keys
Bradley Keys is an accomplished writer who has covered a wide variety of health, nutrition, and wellness topics. He graduated with a Bachelor of Science from Florida State University, and has extensively explored a diverse range of subjects within the realms of health, wellness, and nutritional supplementation, showcasing a broad and in-depth understanding of these interconnected fields.
Reviewed by :

Dr. Majid Sabour
Dr. Majid Sabour, MD, is a renowned expert in medical weight loss and the founder and medical director of Gent's Doctor clinic in Beverly Hills, California. With over 25 years of experience, Dr. Sabour is board-certified in family medicine and specializes in helping patients achieve their weight loss goals through personalized medical treatments. He graduated from Zaporizhzhia State Medical University in Ukraine and completed a family medicine residency program with Columbia University and Cornell at New York-Presbyterian Hospital in Manhattan. Licensed in both New York and California, Dr. Sabour is dedicated to providing comprehensive weight loss solutions that promote overall health and well-being.